

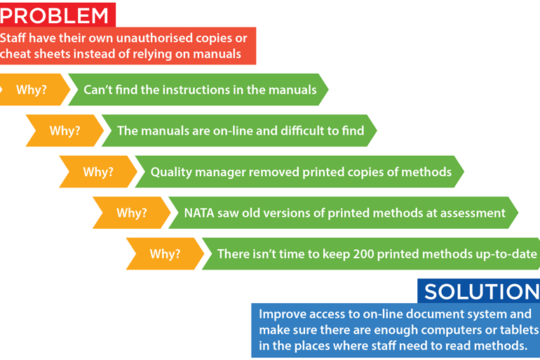
Tools and Tips for NATA-Ready Cause Analysis

From busy to business-mastery
Designed to benefit: Risks & Opportunities in ISO 17025
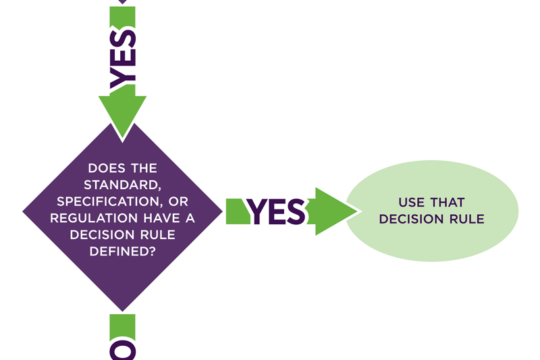
Reporting Compliance in ISO 17025

The 4 Big Benefits of NATA Accreditation

Five steps to implement new accreditation requirements

Bullet-proof your laboratory accreditation

How Safe is Your NATA Accreditation?

The easiest internal audit program ever

Supervision or Teamwork?



