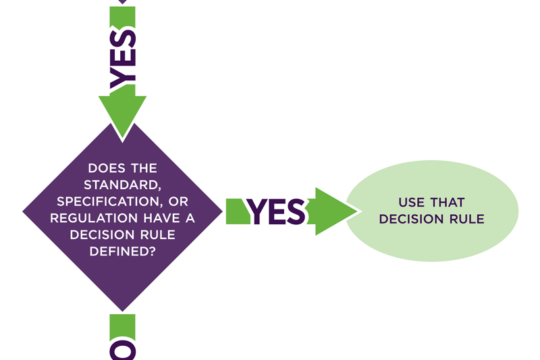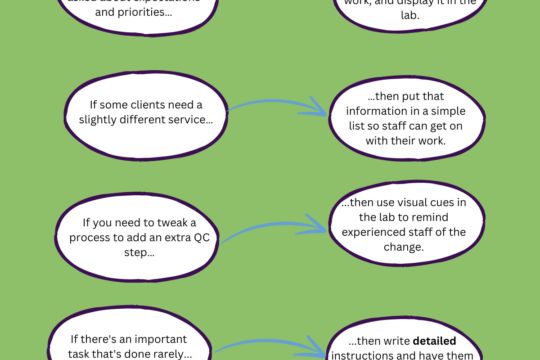
What’s the point of the Quality Management System?

5 simple steps for writing user-friendly lab management documents

How to write great SOPs and declutter lab systems

Why you need a gap analysis

4 Steps to Reducing The Mental Load of Lab Managers

From busy to business-mastery